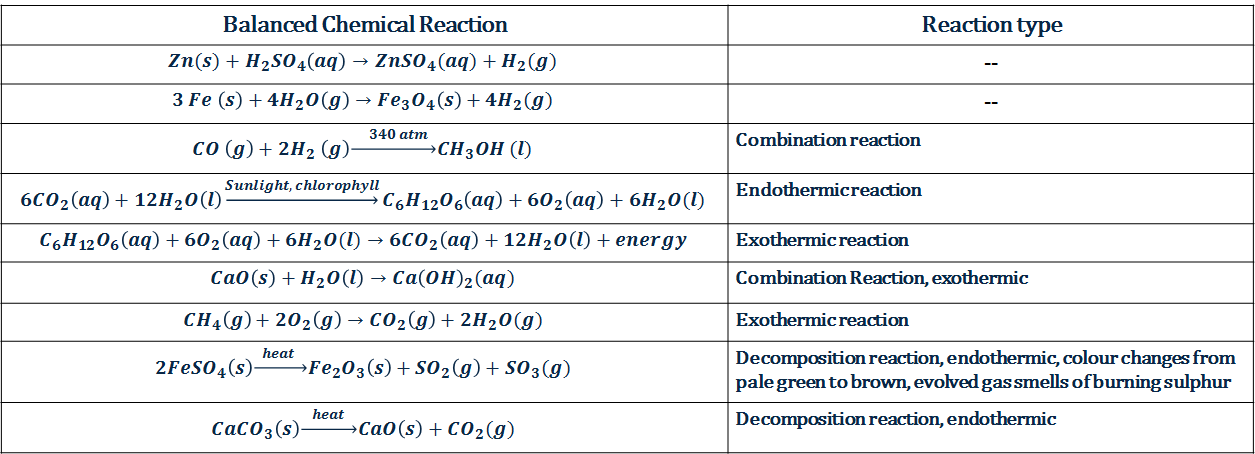
This page will help us revise the first chapter of science – Chemical Reactions and Equations.
This chapter is very important, as the basics of all your future chapters are covered in this chapter.
Let us now look at the weightage of this chapter. On analysing previous years’ papers, we can say that there is a higher probability of a 1 or 2 mark question being asked from this chapter. The questions that have appeared in previous years from this chapter are: